The AGM battery type application
in vehicles is becoming increasingly common because manufacturers equip their
vehicles with technological systems which require huge power supply for their
equipment.
It
should be noted that AGM batteries have become widely used as a result of the introduction
of vehicles fitted with the Start &
Stop system. As of today, most vehicles are equipped with this kind of
batteries due to their great performance.
What
is an AGM battery?
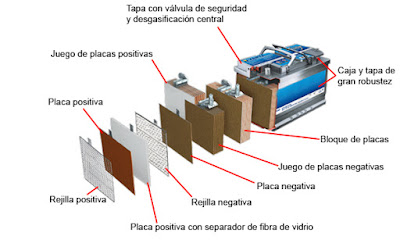
The AGM-type batteries belong
to the VRLA battery family which
stands for Valve Regulated Lead Acid, that is to
say, they are lead acid batteries regulated by valves.
Their constitution is similar to conventional batteries; they are wet lead calcium batteries. The AGM battery
has the same number of positive plates (lead peroxide) and negative plates (sponge
lead) and they provide a 12-Volt
nominal voltage.
The new
aspect of the AGM-type batteries is that they are based on a fibreglass grid placed between the
plates that absorb the electrolyte and the distilled water.
What are its characteristics?
- The electrolyte gases produced during the discharge and charge cycle
are transformed into water.
- The electrodes of the plates fix the fibreglass using pressure, which
means that the fibreglass is always in watertight and uniform contact with the
electrodes. In this way, the loss of
active mass caused by vibrations is minimized.
- In the case of water vapour generation, at high temperature (between 20 and 200 milibars), the system uses an overpressure valve which releases the gas to the atmosphere but prevents the entry of the atmospheric oxygen into the battery.
- The plates are larger in size than the conventional plates.
- The intensity of the energy interchange cycle (discharge and charge) may be up to three times greater due to the fiberglass separators.
- The battery acid is impregnated into the
fibreglass.
TYPE OF BATTERIES
|
|||
TECHNOLOGY
|
LEAD ACID
|
GEL
|
AGM
|
Voltage
|
12 V
|
6V and 12V
|
6V and 12V
|
Battery Capacity (Ah)
|
from 40 to 180
|
from 16 to 210
|
from 33 to 225
|
Cold Start Current EN (A)
|
from 330 to 920
|
from 180 to 1030
|
from 680 to 950
|
Mounting Angle
|
No inclination
|
Any position
|
Any position
|
Charge Requirement
|
DC, 10% of the capacity
|
DC, from 25% to 50% of the capacity
|
DC, any amperage
|
Discharge time
|
After 8 months, 35% of the charge is maintained
|
After 2 years, 85% of the charge is maintained
|
After 2 years, 90% of the charge is maintained
|
Service Life (charge/discharge cycle)
|
Between 350 and 400
|
Between 550 and 600
|
Between 950 and 1000
|
Maximum Discharge Power
|
Around 55% -60%
|
Around 75%
|
100%
|
Possibility of Electrolyte Loss
|
Possible loss of liquid
|
Possible loss of gel
|
None
|
Indicative Data
How does a car battery
work?
Battery Discharging
If a
consumer is connected to the battery, an external electric circuit is established
between the battery terminals due to the potential
difference between both plates and electrodes. The
different nature between them produces
the discharge.
The discharge consists in transforming
the chemical energy to electrical energy. In this way, an electron current
is produced and it circulates from the negative to the positive plate of the
battery at the exterior of the accumulator, and from positive to negative at
the interior (electrolyte).
Remember: The direction of the current is from (+) to (-). This direction is the conventional one, but the real direction is reverse, it is from (-) to (+).
The chemical decomposition occurs as follows:
the sulphuric acid breaks up in sulphate ions and hydrogen ions , the
sulphuric acid of which combines with the plates.
In the positive plate the lead peroxide is decomposed and transformed
into lead sulphate, and oxygen
is released as a result.
In the negative plate, the
sulphate reacts with the lead, so lead sulphate is also
formed. The oxygen
and hydrogen ions released in both reactions combine to form water, which is accumulated inside the
mixture as a part of the battery electrolyte. The electrolyte decomposition (water
+ sulphuric acid) decreases water density.
These
chemical transformations take place inside the battery, and generate electrical
current due to the alteration of the chemical elements between the two plates.
Battery Charging
Charging a battery reverses the chemical process that occurs during the
discharge. This process consists in transforming the electrical energy from a
generator (alternator) into chemical energy. The electrons circulate from the
positive to the negative plate, so a process reverse to the discharge is
produced.
The electrolyte
composition (water + sulphuric acid) increases water density.
The electrolyte water generated
during battery discharge is decomposed by electrolysis in ions (2H+) and (O2). The oxygen
of the water of these ions reacts with the lead of the positive plate. Thus,
the lead peroxide is formed again (PbO2). The
sulphate (SO4=) of both plates is released which makes the
hydrogen react with the sulphate, and sulphuric acid is formed again (H2SO4).
The electrolyte composition (water
+ sulphuric acid) increases water density.
The discharge and charge cycles which are produced inside the battery cause
the deterioration of the chemical elements that form the battery, limiting its
service life as reflected in the number of cycles.






No comments:
Post a Comment